The global Immune
Checkpoint Inhibitors Market by Drug Class (Programmed Death Receptor-1
(PD-1) Inhibitors (Pembrolizumab (Keytruda), Nivolumab (Opdivo), Cemiplimab
(Libtayo), Others (Spartalizumab), Programmed Death-Ligand 1 (PD-L1) Inhibitors
(Atezolizumab (Tecentriq), Avelumab (Bavencio), Durvalumab (Imfinzi)),
Cytotoxic T-Lymphocyte Antigen 4 (CTLA-4) Inhibitors (Ipilimumab (Yervoy)),
Indoleamine-2,3-dioxygenase (IDO) Inhibitors, Lymphocyte-Activation Gene 3
Inhibitors)), By Cancer Type (Lung Cancer, Head & Neck Cancers, Skin Cancer
(Melanoma and Merkel Cell Carcinoma), Blood Cancer (Lymphoma), Bladder Cancer
(Urothelial Carcinoma), Renal/Kidney Cancer, Colorectal Cancer, Breast Cancer,
and Others), by Distribution Channel (Hospital Pharmacies, Retail Pharmacies,
and Online Pharmacies), and Region (North America, Latin America, Europe, Asia
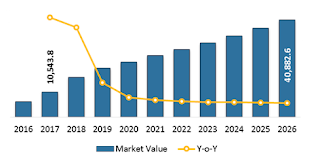
Pacific, the Middle East, and Africa) was valued at US$ 10,543.8 million in
2017, and is projected to exhibit a CAGR of 11.8% over the forecast period
(2018 – 2026).
Increasing prevalence of cancer
is expected to boost the demand for immune checkpoint inhibitors. Also,
innovative drug launches along with robust pipeline is expected to boost the
global immune checkpoint inhibitors market growth over the forecast period.
Major manufacturers are investing into R&D to develop immune-checkpoint
therapies by understanding tumor functions and ways to combat them.
Manufacturers are focusing on upgrading available immune checkpoint inhibitors
as well as developing new immune checkpoint inhibitors for cancer treatment.
For instance, AstraZeneca’s Durvalumab (Imfinzi) was approved in 2017, as
immune checkpoint inhibitor, which blocks interaction of PD-L1 with PD-1 and
CD80. In December 2017, Bristol-Myers Squibb received approval for Nivolumab
(Opdivo) in adjuvant treatment of melanoma. In March 2017, Avelumab (Bavencio),
jointly developed by EMD Serono, and Pfizer, Inc. received U.S. Food & Drug
Administration (FDA) approval for the treatment of metastatic merkel cell
carcinoma. In 2017, the U.S. FDA granted accelerated approval to immunotherapy
product- TECENTRIQ (atezolizumab) for the treatment of patients with locally
advanced or metastatic urothelial carcinoma (mUC).
* The sample copy includes: Report Summary, Table of
Contents, Segmentation, Competitive Landscape, Report Structure, Methodology.
Request a sample copy
of this report: https://www.coherentmarketinsights.com/insight/request-sample/2560
Browse 46 Market Data Tables and
41 Figures spread through 238 Pages and in-depth TOC on Global Immune
Checkpoint Inhibitors Market by Drug Class (Programmed Death Receptor-1 (PD-1)
Inhibitors (Pembrolizumab (Keytruda), Nivolumab (Opdivo), Cemiplimab (Libtayo),
Others (Spartalizumab), Programmed Death-Ligand 1 (PD-L1) Inhibitors
(Atezolizumab (Tecentriq), Avelumab (Bavencio), Durvalumab (Imfinzi)),
Cytotoxic T-Lymphocyte Antigen 4 (CTLA-4) Inhibitors (Ipilimumab (Yervoy)),
Indoleamine-2,3-dioxygenase (IDO) Inhibitors, Lymphocyte-Activation Gene 3
Inhibitors)), By Cancer Type (Lung Cancer, Head & Neck Cancers, Skin Cancer
(Melanoma and Merkel Cell Carcinoma), Blood Cancer (Lymphoma), Bladder Cancer
(Urothelial Carcinoma), Renal/Kidney Cancer, Colorectal Cancer, Breast Cancer,
and Others), by Distribution Channel (Hospital Pharmacies, Retail Pharmacies,
and Online Pharmacies), and region
(North America, Latin America, Europe, Asia Pacific, the Middle East, and
Africa) - Global Forecast to 2025
Research and development by
leading as well as small and mid-sized players in immune checkpoint inhibitors
market is expected to support global immune checkpoint inhibitors market
growth. For instance, Oncolytics Biotech, Inc. announced research collaboration
with the Keck School of Medicine of University of Southern California (USC), in
order to develop a combination therapy of Reolycin (Oncolytics Biotech’s
product), Keytruda, Velcade, and dexamethasone for the treatment of multiple
myeloma in May 2018. Furthermore, in January 2018, Keytruda, developed by Merck
& Co. reported slow liver cancer progression in Phase 2 trial.
Immuno-oncology combination therapies are also under research for various
cancer indications.
Browse Research
Report: https://www.coherentmarketinsights.com/market-insight/immune-checkpoint-inhibitors-market-2560
In January 2018, the U.S. Food
& Drug Administration (FDA) granted breakthrough therapy status to
Lenvima-Keytruda combo for advanced kidney cancer. Furthermore, in February
2018, Opdivo-Yervoy combination therapy showed delayed disease progression in
patients with advanced non-small cell lung cancer. Moreover, Genentech
combination therapy Tecentriq and Avastin delayed kidney cancer progression in
Phase III trials in December 2017. AstraZeneca Plc’s Imfinzi (Durvalumab)
showed delayed non-small cell lung cancer progression in Phase III trials in
November 2017.
Key Takeaways of the Global Immune
Checkpoint Inhibitors Market:
The global immune checkpoint
inhibitors market is expected to exhibit a CAGR of 11.8% over the forecast
period. This is attributed to presence of several leading manufacturers who are
focusing on introducing innovative therapies through extensive research and
development such as Bristol Myers Squibb, Novartis, and Pfizer, Inc.
Biopharmaceutical companies are
developing a robust pipeline of immune-checkpoint inhibitor combination
therapies due to their increasing demand. The U.S. Food & Drug
Administration (FDA) has approved a number of immune checkpoint inhibitors
including Yervoy (anti-CTLA-4), Opdivo and Keytruda (anti-PD1) and Tecentriq
(anti-PD-L1).
Immuno-checkpoint inhibitor
combination therapies are expected to change the market scenario over the
forecast period, owing to positive results in the clinical trials
Research partnerships and collaborations
to develop new drugs by various market players is supporting growth of the
market, as competitors are striving to gain competitive edge in the market
Major players operating in the
global immune checkpoint inhibitors market include Bristol-Myers Squibb
Company, Merck & Co., Inc., F. Hoffmann-La Roche AG, AstraZeneca Plc.,
Novartis International AG, ImmunOs Therapeutics AG, Immutep Ltd., NewLink
Genetics Corporation, Ono Pharmaceutical Co., Ltd., and Pfizer, Inc.
Buy-Now this research
report: https://www.coherentmarketinsights.com/insight/buy-now/2560
About
Coherent Market Insights:
Coherent
Market Insights is a prominent market research and consulting firm offering
action-ready syndicated research reports, custom market analysis, consulting
services, and competitive analysis through various recommendations related to
emerging market trends, technologies, and potential absolute dollar
opportunity.
Contact
Us:
mailto:sales@coherentmarketinsights.com
U.S.
Office:
Name: Mr. Shah
Coherent
Market Insights 1001 4th Ave,
# 3200
Seattle, WA 98154, U.S.
US : +1-206-701-6702
UK : +44-020-8133-4027
JAPAN : +050-5539-1737
No comments:
Post a Comment